|
BACKGROUND
Glioblastoma (GBM) is the most common and aggressive brain cancer, and accounts for 60% of adult brain tumours. The median survival of adult GBM patients is 15 months, where the standard-of-care is a combination of surgery, radiation, and chemotherapy using the DNA alkylating agent temozolomide (TMZ), which extends patient survival by approximately two months.
However, TMZ is not cancer cell specific, resulting in neural, hematological, and gastrointestinal toxicities. 50% of patients do not respond to TMZ due to the tumour’s heterogeneity. Moreover, 90% of patients develop recurrence, where there is no standardized care for recurrent GBM, and the median survival is less than six months.
This highlights the demand to develop a therapy that is effective and cancer cell specific. Here, we describe an invention that targets a non-conventional molecular target specific to GBM cells.
DESCRIPTION OF THE INVENTION
Ion channels control ion flow across cell membranes, contributing to the regulation of biological processes. Dr. Xi Huang's (The Hospital for Sick Children) previous publications1–5 identified the role of ion channels in mediating mechano-electrical-chemical signalling in tumour cells, regulating tumour growth and metastasis, and in hand, driving glioblastoma aggression.
The research team recently discovered a novel ion channel complex expressed only in GBM cells that promotes tumour growth. Due to its absence in non-tumour cells, the group targeted this interaction using a designer peptide as a therapy for GBM. Using an orthotopic xenograft model, the designer peptide was administered through cannula mediated delivery, precisely killing GBM cells and improving overall survival of the mice.
Furthermore, using xenograft models derived from TMZ-resistant GBM patient-derived cells, the designer peptide showed robust efficacy in killing TMZ-resistant GBM cells and significantly improved overall survival of the mice.
COMMERCIAL APPLICATIONS & ADVANTAGES
As the designer peptide targets a novel ion-channel complex that is uniquely present in tumour cells but not non-tumoural cells, it provides GBM-targeting specificity that is absent in the current standard-of-care. No side effects were detected in all preclinical mouse model studies.
Moreover, it addresses 50% of GBM patients who do not respond to TMZ. These findings support the potential of the designer peptide in:
- Replacing TMZ for treating primary GBM
- Replacing TMZ and other chemotherapeutic drugs for treating recurrent GBM
- Treating other tumour types in which this ion-channel complex is expressed (i.e., medulloblastoma)
DEVELOPMENTAL STAGE
- In vivo assessment of designer peptide in TMZ-sensitive and TMZ-resistant GBM is complete.
- In vivo assessment in post-therapy recurrent GBM is complete.
- PK/PD study is near completion.
PATENT STATUS
A PCT patent application covering methods and composition of matter has been filed.
PUBLICATION
- Huang, X. et al. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Genes Dev 26, 1780–1796 (2012).
- Huang, X. et al. EAG2 potassium channel with evolutionarily conserved function as a brain tumor target. Nat Neurosci 18, 1236–1246 (2015).
- Chen, X. et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 100, 799–815 e797 (2018).
- Francisco, M. A. et al. Chloride intracellular channel 1 cooperates with potassium channel EAG2 to promote medulloblastoma growth. J Exp Med 217 (2020).
- Chen, X. et al. Mechanosensitive brain tumor cells construct blood-tumor barrier to mask chemosensitivity. Neuron 111, 30–48 e14 (2023).
|
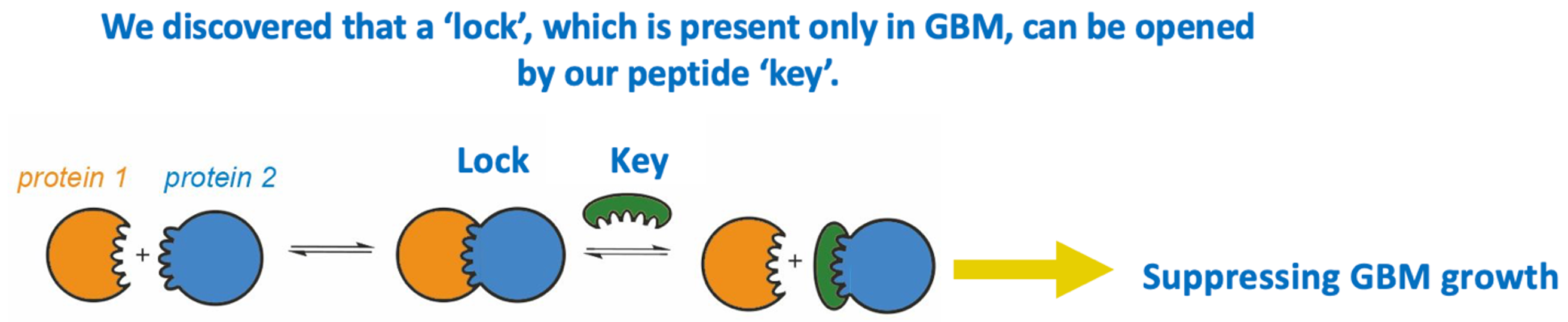 Figure 1. We discovered a unique ion channel complex which is required for GBM to grow. We engineered a designer peptide to disrupt the assembly of this ion channel complex, thereby effectively impeding GBM growth.
Figure 1. We discovered a unique ion channel complex which is required for GBM to grow. We engineered a designer peptide to disrupt the assembly of this ion channel complex, thereby effectively impeding GBM growth.
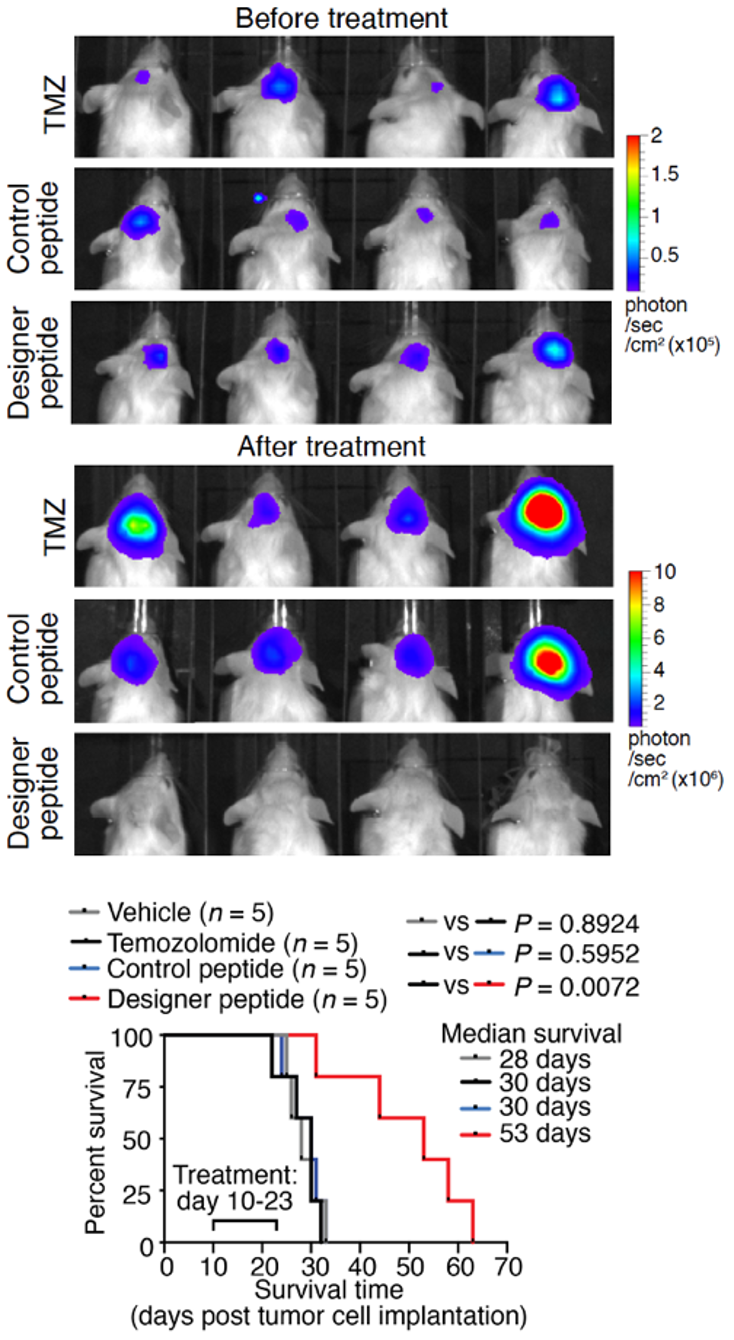 Figure 2. Treatment with designer peptide in mice bearing patient-derived GBM tumours demonstrated our designer peptide’s robust therapeutic efficacy in mitigating TMZ-resistant GBM.
Figure 2. Treatment with designer peptide in mice bearing patient-derived GBM tumours demonstrated our designer peptide’s robust therapeutic efficacy in mitigating TMZ-resistant GBM.
|