|
BACKGROUND
Clostridium difficile (C. difficile), a spore-forming bacteria, is the leading cause of hospital acquired diarrhea and antibiotic associated pseudomembranous colitis. Disruption of the protective gut microbiota by antibiotics enables colonization by multidrug resistant C. difficile, which secrete up to three different protein toxins that are responsible for the gastrointestinal sequelae. Infections resulting from C. difficile present a significant health burden, costing upwards of $4.5 billion. Disease symptoms associated with infection arise from the actions of secreted virulence factors, including two homologous glycosylating toxins TcdA, TcdB, and an unrelated ADP-ribosylating binary toxin. Recent studies highlighting the role of each individual toxin in disease suggest that inhibiting the actions of all three toxins is required for optimal protection against disease and recurrence associated with C. difficile infections.
DESCRIPTION OF THE INVENTION
The Melnyk lab at SickKids have shown that an FDA approved off patent tapeworm therapeutic, niclosamide inhibits the actions of all three toxins, by modifying the host cells with its mechanism of inhibiting endosomal acidification, an essential step required for cytosolic entry and toxicity by all three toxins. In contrast to other endosomal acidification inhibitors, however, which accumulate in acidic compartments and are typically toxic to host cells and disrupt beneficial gut microbiota at doses that inhibit toxin uptake, SickKids researchers have shown that niclosamide is nontoxic and not disrupting beneficial gut microbiota at efficacious doses. In addition, niclosamide was shown to be effective in blocking a more virulent form of TcdB expressed by NAP1/027/BI which escapes endosomes at an earlier step in endocytosis.
COMMERCIAL APPLICATIONS & ADVANTAGES
- Nicoslamide is well characterized as a previously FDA-approved therapeutic in tapeworm infection and is off- patent.
- Niclosamide is non-toxic and not disrupting beneficial microbiota at efficacious doses.
- Recent monoclonal antibodies have been developed for TcdA/B but are ineffective following infection onset.
- Niclosamide’s distinctive mechanism of action of inhibiting endosomal acidification provides a broader therapeutic opportunity to treat pathogenic infections that require endosomal acidification as an essential step for endocytosis.
DEVELOPMENT STAGE
Completed in vitro and in vivo safety assays.
PATENT STATUS
Patent is granted in USA: Composition and method for protecting a host from enteric toxigenic pathogens. Patent No.–US 11,166,926 B2. Date of Patent–Nov 9, 2021.
Patent application is filed in Canada.
ADVANTAGES OF THE INTELLECTUAL PROPERTY
- Granted claims are broad on the use of the salicylanilide class of compounds including niclosamide ethanolamine salt to treat (stand alone, or in combination with antibiotics as 1L, 2L) a host infected with or at risk of infection with a pathogen including C. Difficile bacteria.
- The granted broad claims are based on SickKids researchers’ original invention describing the mechanism of action of niclosamide to modify the host cells, inhibit endocytosis of C. Difficile toxins, and hence to prevent infection. This IP establishes the foundation for using the salicylanilide class of compounds (e.g., niclosamide) to treat and prevent infection of a broad class of pathogens.
PUBLICATION
Melnyk et al. 2018. Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota. Nature Communications. 9: 5233.
|
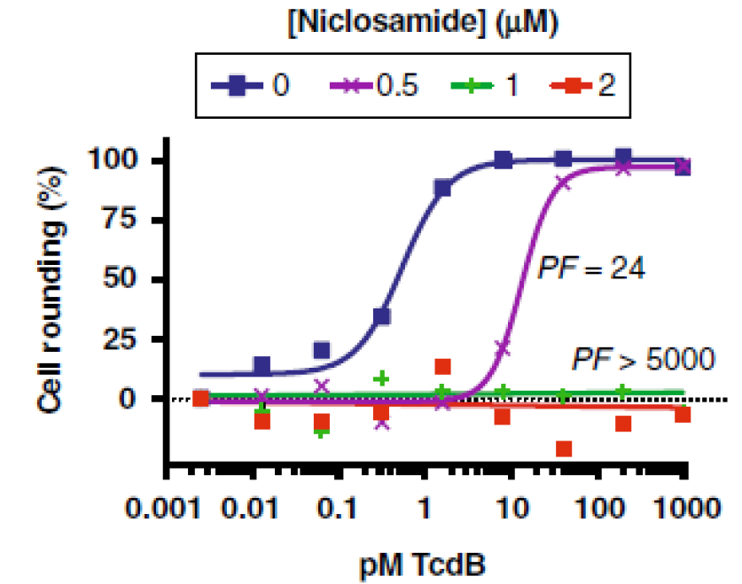 Figure 1. Effect of Niclosamide on inhibiting C. Difficile TcdB toxin induced cell rounding in human IMR-90 fibroblasts. Fibroblasts were exposed to different TcdB concentrations along with fixed concentrations of niclosamide for 3h.
Figure 1. Effect of Niclosamide on inhibiting C. Difficile TcdB toxin induced cell rounding in human IMR-90 fibroblasts. Fibroblasts were exposed to different TcdB concentrations along with fixed concentrations of niclosamide for 3h.
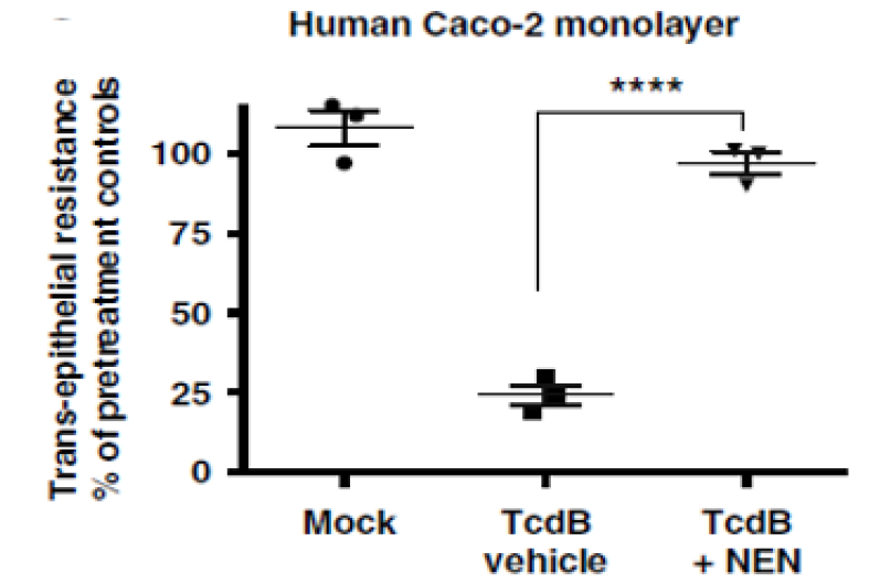 Figure 2. Niclosamide ethanolamine salt prevented the TcdB-induced disruption of colorectal cells (Caco-2 monolayers) maintaining barrier function to untreated levels
Figure 2. Niclosamide ethanolamine salt prevented the TcdB-induced disruption of colorectal cells (Caco-2 monolayers) maintaining barrier function to untreated levels
|