|
BACKGROUND
Unpredicted cardiotoxicity and cardiovascular safety concerns are responsible for the termination of nearly one-third of all compounds in drug discovery pipelines. These unforeseen adverse effects add significant cost and time to therapeutic discovery and expose patients to an unneeded risk of harm.
Current in vitro and in vivo preclinical models of cardiac function inadequately predict how a drug candidate will affect cardiac activity or induce toxicity. Elimination of compounds with potential cardiotoxicity earlier in the drug discovery continuum will save substantial time and cost of drug development. SickKids clinician researcher Dr. Jason Maynes and his collaborator Dr. Yu Sun at the University of Toronto have developed a high-throughput in vitro cardiotoxicity assay platform that integrates machine learning algorithms to quantify drug candidate cardiotoxicity and provide insight into potential mechanisms and intracellular targets.
DESCRIPTION OF THE INVENTION
Our developed cardiotoxicity assay platform, MATCH (Machine Learning Algorithms for Toxicity and Cardiac Health), includes a patented carbon nanotube sensor device and novel computer vision assays for the continuous measurement of functional human cardiac tissue activity, with proprietary machine learning algorithms for data analysis and reporting (Figure 1). The platform incorporates multiple tests of cardiac function into a unified, multi-parametric system, providing superior predictive power. The use of non-biased machine learning algorithms allows the platform to determine which metrics are the most predictive of a drug candidate’s effect on the human heart and provides insight into cellular targets/mechanism, eliminating unnecessary tests and setting an industry standard for in vitro cardiac activity screening.
MATCH is supported by the following analyses and more.
1. Beating and Contractility Analysis
Our approach provides a comprehensive determination of tissue function, including contractility, beating rate and rhythm, and electromechanical coupling, using a patented carbon nanotube sensor and soft substrate optical assay. These systems were developed specifically to analyse drug effect on cardiac tissue composed of induced pluripotent stem cell derived cardiomyocytes (“iPSC-CMs”), which has been shown to be the first platform that recapitulates the in vivo effect of cardioactive drugs.
2. Real-time Tissue Stiffness Analysis
MATCH can determine how a compound alters tissue function in the context of a healthy (soft) and diseased (stiff, fibrotic) heart, using imaging and optical flow analysis to determine changes to cardiomyocyte function and calcium transients. This analysis is unique and illustrates how drug effect is dependent on the context of organ health.
3. Drug Candidate Classification and Toxicity Prediction
The above parameters are integrated through a proprietary machine-learning classifier trained on the response of pharmaceuticals of known in vivo activity (Figure 2). This analysis provides the customer with a predictive mechanism of action and informs on potential molecular targets for a drug candidate.
Our MATCH analysis has successfully identified the cardiac activity or toxicity of unknown investigational compounds, providing mechanistic insight to customers. We generate a comprehensive user-friendly report that outlines the drug effect on each activity parameter, as well as predictive categorization, without a priori knowledge of the drug to ensure the security of customer intellectual property.
The human cardiomyocyte performs a complex set of tasks, including the initiation/conduction of an electrical signal that results in regular beating (beat rate and rhythm) and generating a force of contraction for the expulsion of blood from the heart (contractility). Currently available pre-clinical analysis systems measure only some of these functions, resulting in a poor estimation of drug effect on cardiomyocyte function, and often use non-human tissue (unreliable translation to human in vivo effect) (Figure 3). MATCH is the first commercial platform to comprehensively measure cardiomyocyte function in human-derived tissue, with a throughput amenable to pre-clinical pipeline compound analysis.
COMMERCIAL APPLICATIONS & ADVANTAGES
MATCH integrates multiple predictive assays to evaluate the cardiotoxicity profile of investigational drugs and generates a single machine learning-based report designed to seamlessly integrate into contemporary preclinical drug discovery hit-to-lead validation. It provides a robust cardiotoxicity profile unparalleled in the industry, translating to reduced cardiotoxicity of advanced drug candidates, lowering pipeline drug attrition, and improving clinical trial success through better predictive patient safety.
DEVELOPMENT STAGE
Our proprietary assays are patented, and our predictive algorithms are validated using 16 different classes of drug compounds with a known effect on the human heart including: inotropic ions, calcium sensitizers, beta-adrenoceptor agonists, beta-adrenoceptor antagonists, PDE5 inhibitors, SERCA2A antagonists, ryanodine receptor antagonists, funny channel antagonists, calcium channel antagonists (dihydropyridine), calcium channel antagonists (non-dihydropyridine), local anesthetics, hERG antagonists, Nav1.5 antagonists, myosin inhibitors, anthracyclines, and cardiotropic hormones. For each compound, we collect over 40 distinct metrics for quantification, comparison, and categorization. We are currently able to provide drug candidate analyses for hundreds of compounds at a time.
|
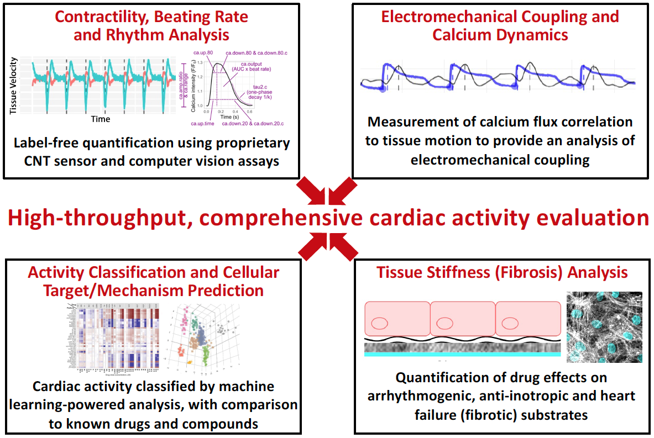 Figure 1. Overview of the MATCH assay platform and analysis
Figure 1. Overview of the MATCH assay platform and analysis
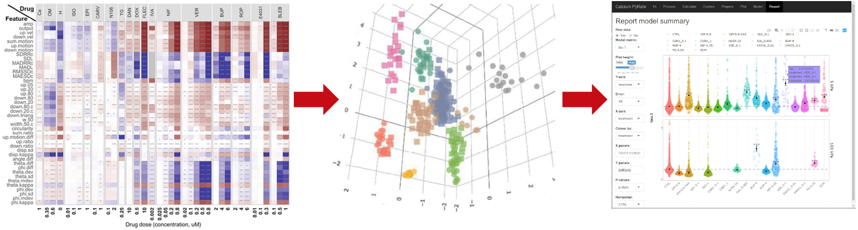 Figure 2. Drug classification and categorization with putative cellular target identification involving assay response collection (left), drug categorization in ML classifier (middle) and customer report illustrating functional metrics (right)
Figure 2. Drug classification and categorization with putative cellular target identification involving assay response collection (left), drug categorization in ML classifier (middle) and customer report illustrating functional metrics (right)
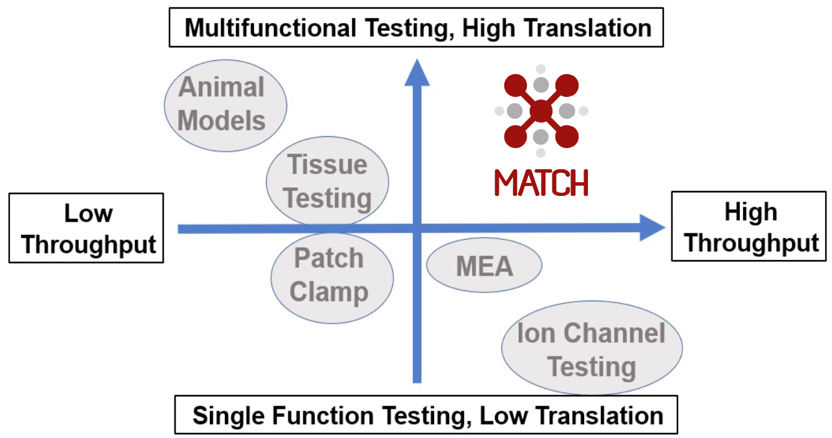 Figure 3. The pre-clinical cardiac drug testing paradigm currently has a trade-off between throughput and clinical translation/accuracy. MATCH analysis offers the throughput needed for pre-clinical pipeline hit-to-lead compound evaluation with high translation to human in vivo effect
Figure 3. The pre-clinical cardiac drug testing paradigm currently has a trade-off between throughput and clinical translation/accuracy. MATCH analysis offers the throughput needed for pre-clinical pipeline hit-to-lead compound evaluation with high translation to human in vivo effect
|