BACKGROUND
Chronic obstructive pulmonary disease (COPD) is induced by environmental toxins and severely compromises breathing. It is associated with chronic bronchitis with obstruction of the large and small airways leading to diminished airflow. Currently, treatments for COPD aim to manage symptoms through the use of bronchodilators to improve airflow and antibiotics to eradicate pulmonary infections. However, despite these interventions, the disease is progressive rendering patients vulnerable to severe outcomes after COVID-19 infection and exposure to smoke from forest fires.
The CFTR chloride channel is situated on the luminal membrane of airway surface epithelium and serves to enhance the fluidity of the airway surface liquid, thereby preventing mucus obstruction. Tobacco smoke reduces the functional expression of CFTR on the epithelial surface, exacerbating mucostasis and obstruction. These findings support the hypothesis that smoke exposure induces CFTR deficiency, also known as “acquired Cystic Fibrosis” and small molecules that potentiate activity of the remaining CFTR function, will reverse the initial stages of COPD pathogenesis. In fact, Ivacaftor, an approved potentiator of CFTR was shown to reverse mucostasis in certain tissue culture models and a novel CFTR potentiator called Icenticaftor has shown early promise in restoring lung function in clinical trials of people with COPD (5,6).
DESCRIPTION OF INVENTION
We have discovered novel small molecule CFTR potentiators. Recently, the leading SickKids potentiator (SK 9919) was tested in an in-vitro model of COPD. In this model, primary differentiated human bronchial epithelial cultures were exposed to cigarette smoke extract. We confirmed that exposure to cigarette smoke extract (CSE) led to mucostasis and slowed mucociliary movement. We found that the SickKids compound prevented the mucostasis induced by cigarette smoke extract when added to differentiated airway epithelial cultures. Changes in mucociliary movement were determined by tracking the speed of fluorescent beads added to the surface of the airway cultures. The relative rescue effect of the SickKids potentiator compared favourably relative to the competitor, the FDA approved CFTR potentiator marketed as Ivacaftor (VX-770).
MARKET OPPORTUNITY
According to the CDC, the monetary market size of COPD was valued at USD 10.17 Billion in 2018 and is projected to reach USD 13.9 Billion by 2026.
Challenge Gap: The current treatments, including bronchodilators and antibiotics are modestly effective in slowing the progressive deterioration of lung function and there are few novel therapeutic targets being interrogated. Ideally, interventions that target mucostasis and mucus obstruction, considered as initial steps in the pathogenesis of COPD, would significantly slow disease progression, improve health outcomes and alleviate costs to the health care system. Our discovery of a novel class of CFTR potentiator provides the opportunity to directly target mucostasis and slow disease progression.
Limitation of current solutions: The COPD market is becoming saturated with anti-infective agents that prevent exacerbations, but there is no clear data pertaining to improved mortality outcomes. The two biologics in the COPD pipeline, GSK’s Nucala (mepolizumab) and AstraZeneca’s benralizumab, are the only therapies offering a novel mechanism of action targeting inflammation. An oral potentiator of CFTR called Icenticaftor (developed by Novartis/Enterprise Therapeutics), has been shown to ameliorate mucostasis in preclinical models of COPD and improve lung function in Phase 2 clinical trials. However, there are side effects associated with this oral CFTR potentiator, including diarrhea.
Unmet need addressed by SickKids potentiators: Our recently discovered class of CFTR potentiator is potent, highly effective and amenable to delivery by inhalation and hence, less likely to mediate side effects (such as diarrhea) associated with Icenticaftor.
DEVELOPMENT STAGE
Our key compound has demonstrated effectiveness in ameliorating the deleterious effect of cigarette smoke extract on mucocilliary clearance. This sets the stage for future evaluation in the ferret model of COPD and eventually advancement as a therapeutic candidate for this life limiting disease.
PATENT STATUS
A US Provisional patent application has been filed for Compounds Which Modulate Mutant Proteins for Treating Respiratory Diseases.
|
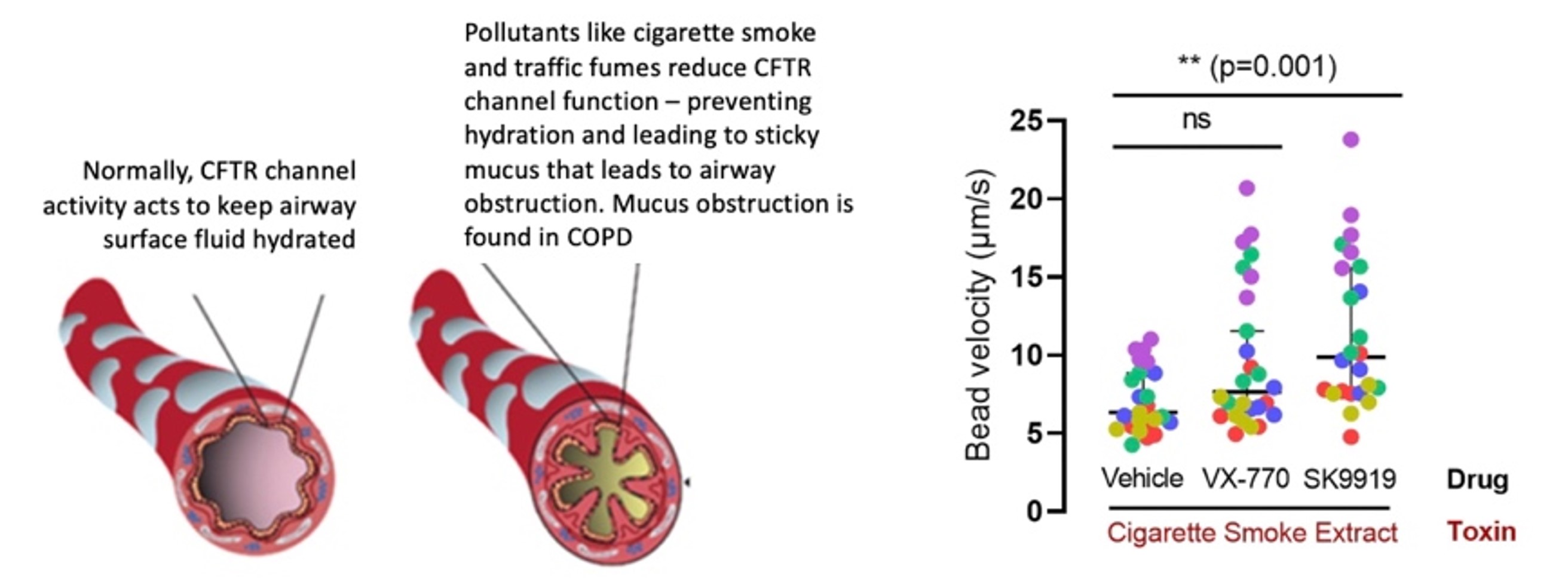 SickKids CFTR potentiator (SK 9919) enhances mucociliary movement on the surface of primary bronchial cultures previously exposed to cigarette smoke extract. In the figure on the left, we show the accumulation of sticky mucus that occurs in the airways of people with CFTR mutations or in patients with COPD. The graph on the right, we show our studies of mucus rheology on the surface of human bronchial cultures. We studied cultures from five different lung transplant donors. Cultures were exposed to cigarette smoke extract and pretreated with vehicle (DMSO), Ivacaftor (VX-770) or our asset, SK 9919 for 24 hours. Those cultures pretreated with the SK-9919 potentiator, showed a significant increase in bead velocity—the in-vitro measure of improved hydration and mucociliary movement. The increase in bead velocity with the Vertex compound (VX-770) was not statistically significant. Each symbol represents the mean bead velocity in five videos taken at randomly selected regions on each airway culture. Each colour corresponds to data from a particular donor.
SickKids CFTR potentiator (SK 9919) enhances mucociliary movement on the surface of primary bronchial cultures previously exposed to cigarette smoke extract. In the figure on the left, we show the accumulation of sticky mucus that occurs in the airways of people with CFTR mutations or in patients with COPD. The graph on the right, we show our studies of mucus rheology on the surface of human bronchial cultures. We studied cultures from five different lung transplant donors. Cultures were exposed to cigarette smoke extract and pretreated with vehicle (DMSO), Ivacaftor (VX-770) or our asset, SK 9919 for 24 hours. Those cultures pretreated with the SK-9919 potentiator, showed a significant increase in bead velocity—the in-vitro measure of improved hydration and mucociliary movement. The increase in bead velocity with the Vertex compound (VX-770) was not statistically significant. Each symbol represents the mean bead velocity in five videos taken at randomly selected regions on each airway culture. Each colour corresponds to data from a particular donor.
|