|
BACKGROUND
Sudden cardiac arrest (SCA) is the abrupt loss of heart function, breathing, and consciousness. SCA is usually caused by an electrical disturbance in the heart, which disrupts heart pumping and consequently the blood flow. In the case of a defect in electrical impulses or in the sinus node, SCA can lead to abnormal heart rhythm or arrhythmia. If not treated immediately, SCA can lead to Sudden Cardiac Death (SCD), which is the second largest cause of life-years lost in the US. Some SCD disorders are difficult to diagnose, such as Brugada Syndrome (BrS).
BrS is a heritable disorder associated with an increased risk of SCD from ventricular arrhythmias and is characterized by a unique ECG pattern of coved “ST” segment elevation (>2 mm) followed by a negative T wave in anterior chest leads (called the Type 1 Brugada Syndrome pattern). BrS causes 4–12% of SCD, where 20% of the patient hearts are structurally normal. The world prevalence is 1 in 2,000 persons, but it is much higher in those of southeast Asian descent. Once diagnosed, patient management includes alcohol avoidance and implantation of a defibrillator for high-risk cases.
DESCRIPTION OF THE INVENTION
The Hamilton lab has discovered a biomarker profile of autoantibodies against 4 cardiac proteins—alpha-cardiac actin, alpha-skeletal actin, keratin, and connexin-43. These autoantibodies can be identified from sera of BrS patients, using a novel method that is highly sensitive, specific, and independent of the genetic cause of BrS.
COMMERCIAL APPLICATIONS & ADVANTAGES
Genetic testing for SCN5A mutations is only performed in patients with a likelihood of developing BrS. However, mutations in SCN5A are only found in 11–28% of BrS patients. Clinical diagnosis is costly and is based on identification of the typical ECG pattern, which is often transient. Our researchers’ profile of biomarkers identifies 100% of probable cases that develop into BrS, with possible commercial applications of:
- Development as a diagnostic and prognostic biomarker
- Predictive testing of disease progression, which is in development
DEVELOPMENT STAGE
ELISA assay is being developed by a CRO to research use only standards, and researchers are currently collaborating with all Canadian Inherited Arrhythmia Clinics and major US and European centres.
PATENT STATUS
Patent application filed Canada, US, Korea, and Japan.
|
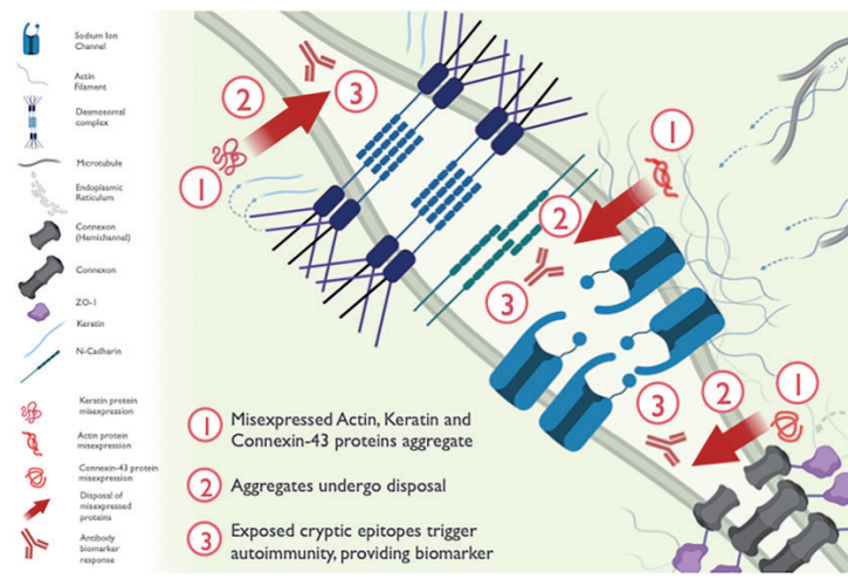 Figure 1. Summary of biomarker profile for Brugada syndrome
Figure 1. Summary of biomarker profile for Brugada syndrome
|