|
BACKGROUND
Helicobacter pylori (H. pylori) infects the stomach of half of the world’s population. Infection causes peptic ulcer disease, is associated with functional dyspepsia and is recognized by the World Health Organization (WHO) as a carcinogen that causes gastric cancer; the third leading cause of cancer-related deaths worldwide. As eradication of H. pylori reduces gastric cancer, the WHO has called for H. pylori screening and treatment programs to reduce cancer risk.
The current treatment for H. pylori uses a combination of antibiotics and proton pump inhibitors (OMC). However, these treatments fail to reach the recommended eradication rates due to increasing antibiotic resistance. Furthermore, there are no treatments targeting the pool of bacteria living inside gastric cells that serve as a reservoir for persistent infections.
Approximately 60% of H. pylori strains produce a virulence factor, vacuolating cytotoxin (VacA), that promotes infection and causes more severe disease, including gastric cancer.
DESCRIPTION OF THE INVENTION
SickKids researcher Dr. Nicola Jones, and her team, discovered that VacA inhibits a protein called TRPML1 to generate the intracellular niche in vivo that protects the bacteria from antibiotic treatment and leads to infection recrudescence after therapy. Activating TRPML1 reverses VacA toxic effects and kills the intracellular bacteria. Furthermore, they have identified and selected two novel small molecules (named “G” and “H”) that activate TRPML1, eradicate intracellular H. pylori in infected cells, and control the progression of infection in mice.
COMMERCIAL APPLICATIONS & ADVANTAGES
SickKids researchers identified two small molecule agonists of TRPML1 able to treat H. pylori infection in a mouse model without toxicity. Importantly, one of the compounds is a gut-restricted formulation with no systemic absorption that was optimized for orogastric administration.
DEVELOPMENTAL STAGE
- In vivo assessment of the novel small molecules in mouse models of H. pylori infection is complete.
- The gut-restricted compound is 12 months from an IND submission.
- In vivo experiments to optimize the dosing regimen and confirm target specificity are underway.
- Upon completion of the above, we will have defined the appropriate formulation and dosing regimen for a preclinical TRPML1 small molecule agonist that effectively eradicates H. pylori infection without toxicity and off-target effects.
PUBLICATIONS
Nature Microbiology 2019 August: 4(8): 1411–1423
PATENT STATUS
US Pat. # 11,191,760 issued Dec 7, 2021
CA Pat. # 3058679 filed Apr 6, 2017
US Pat Appl. # 17/515706 filed Nov 1, 2021
|
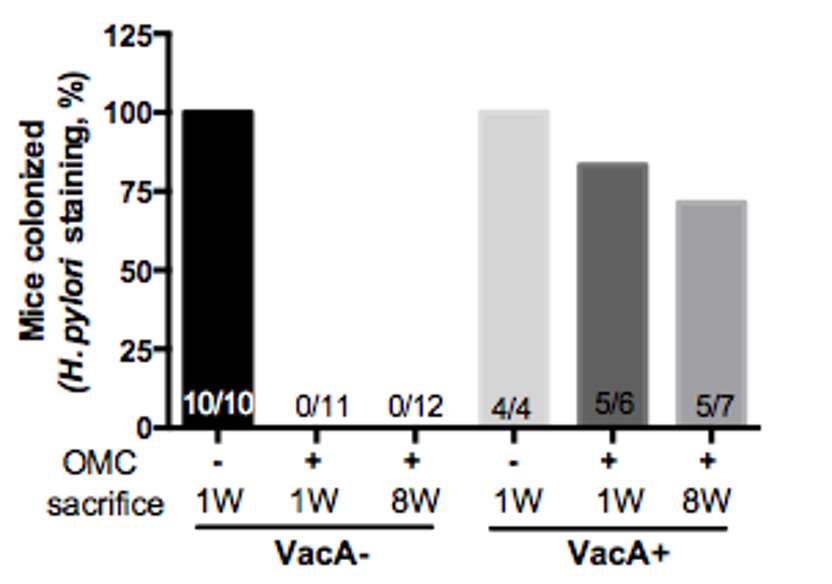 Figure 1. VacA causes persistent infection after standard eradication therapy. Proportion of mice positive for H. pylori staining 1 week (1W) and 8W after eradication therapy (OMC). VacA- bacteria were completely eradicated by OMC but VacA+ H. pylori escapes eradication.
Figure 1. VacA causes persistent infection after standard eradication therapy. Proportion of mice positive for H. pylori staining 1 week (1W) and 8W after eradication therapy (OMC). VacA- bacteria were completely eradicated by OMC but VacA+ H. pylori escapes eradication.
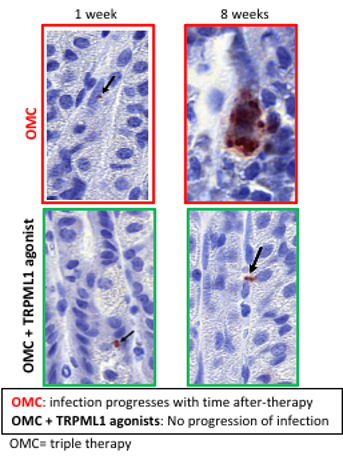 Figure 2. TRPML1 agonists prevent persistent infection. Representative H. pylori staining of gastric mucosa obtained from H. pylori–infected mice treated with standard triple therapy (OMC) alone or in combination with TRPML1 agonist compound. Mice were sacrificed 1 week or 8 weeks after treatment.
Figure 2. TRPML1 agonists prevent persistent infection. Representative H. pylori staining of gastric mucosa obtained from H. pylori–infected mice treated with standard triple therapy (OMC) alone or in combination with TRPML1 agonist compound. Mice were sacrificed 1 week or 8 weeks after treatment.
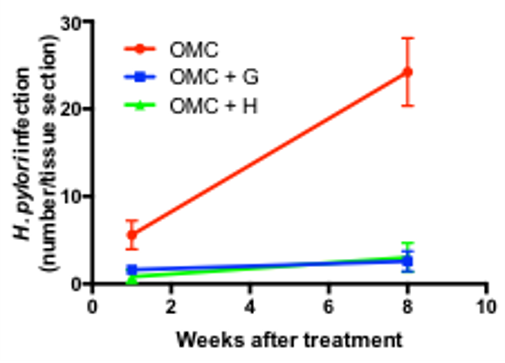 Figure 3. No progression of the infection with TRPML1 agonist. H. pylori counts for each treatment at the different times of sacrifice. Mean +/- SD, 5 animals/group.
Figure 3. No progression of the infection with TRPML1 agonist. H. pylori counts for each treatment at the different times of sacrifice. Mean +/- SD, 5 animals/group.
|